

Higher and foundation tiers
Many of the physical properties of the alkanes depend on the size of the particular alkane molecule,
these properties include: boiling and melting points,
viscosity and flammability. Now
viscosity is simply how runny or how easily a substance will flow and flammability
which is how readily
a substance catches fire. These properties are particularly important for
hydrocarbons since their main use is as fuels.
| alkane | molecular formula | boiling point/0C | melting point point/0C | state at room temperature |
|---|---|---|---|---|
| methane | CH4 | -161 | -182 | gas |
| ethane | C2H6 | -88 | -183 | gas |
| ethane | C2H6 | -88 | -183 | gas |
| propane | C3H8 | -42 | -188 | gas |
| butane | C4H10 | -0.5 | -138 | gas |
| pentane | C5H12 | 36 | -130 | liquid |
| hexane | C6H14 | 69 | -95 | liquid |
| heptane | C7H16 | 98 | -90 | liquid |
| octane | C8H16 | 126 | -57 | liquid |
| nonane | C9H20 | 151 | -51 | liquid |
| decane | C10H22 | 174 | -30 | liquid |
The trends in the boiling point and melting points is easy to spot from the table; the larger the alkane molecule the higher the boiling and melting points. This trend is probably what you were expecting:
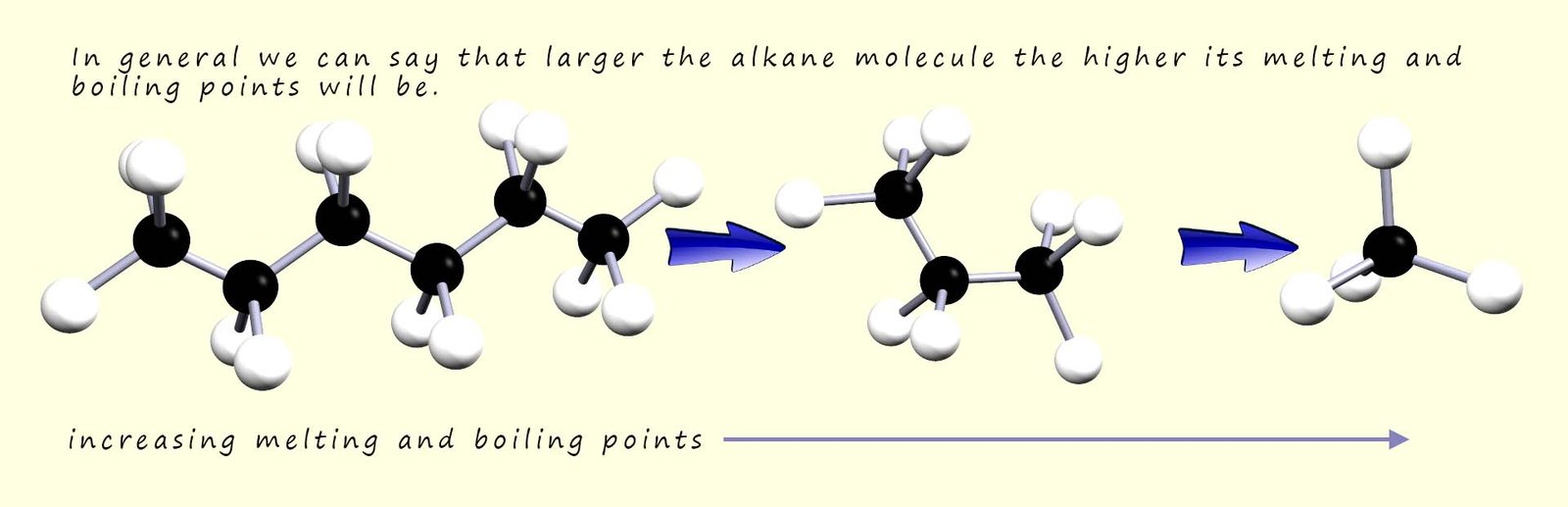
The larger the alkane molecule the larger it's molecular mass and the more intermolecular bonding there is between different molecules; so its boiling and melting points both increase. This is outlined in the diagram below; where the two alkane molecules ethane and butane are shown. Butane being a larger molecule than ethane has more intermolecular bonding present between different butane molecules.
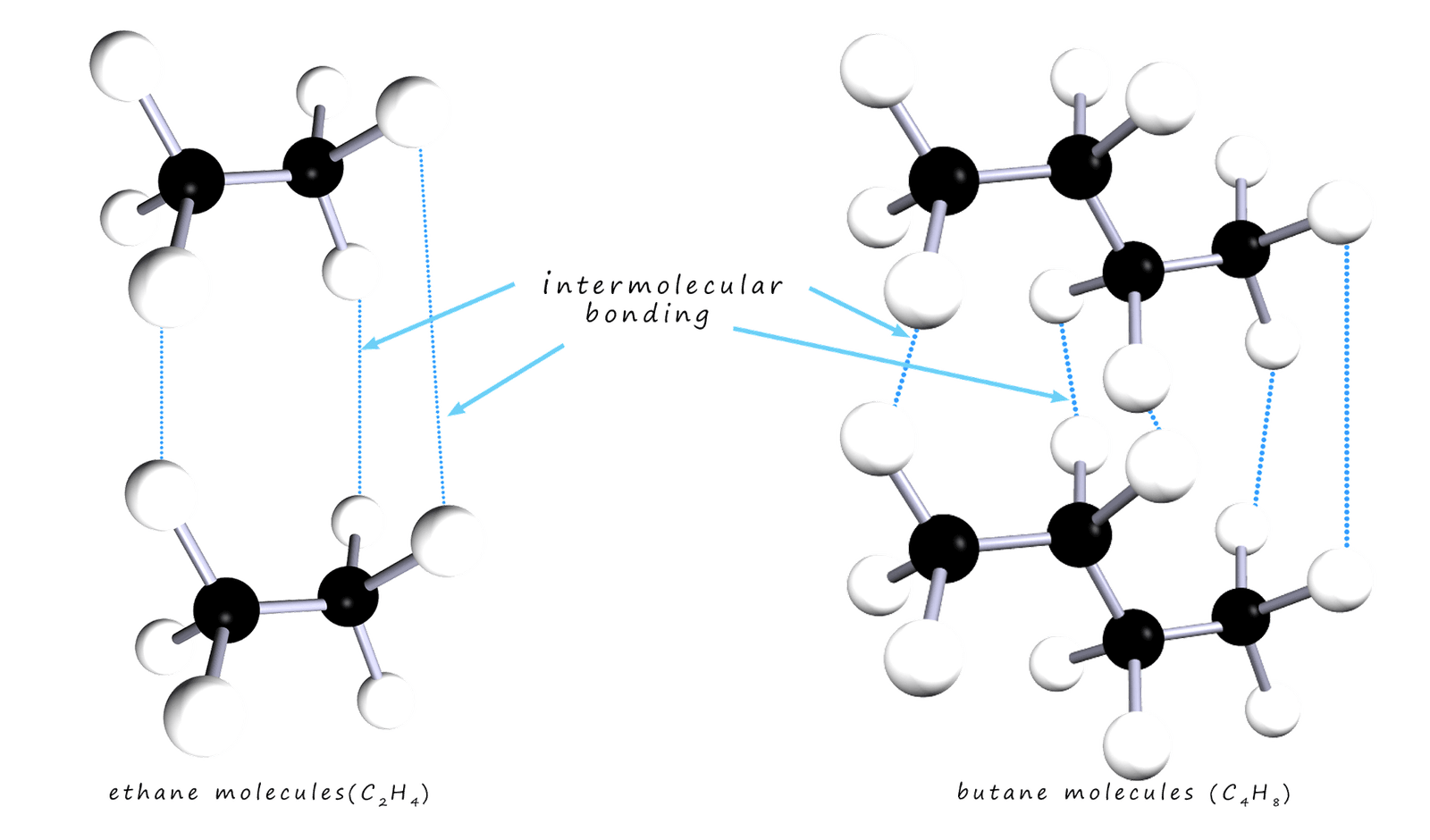
The general trends is similar for the melting points, the first 4 alkanes are all gases at room temperature while the larger alkane molecules from pentane (C5H12) to hexadecane (C16H34) are all liquids at room temperature. The viscosity of the alkane liquids also changes with chain length; the shorter the chain length the more runny and the more easily it flows, that is it is less viscous. The longer the carbon chain the more viscous and thick is the liquid alkane.
A volatile substance is one that evaporates easily. The smaller the alkane molecule the lower will be its boiling point and being a small molecule will mean there is only a small amount of intermolecular bonding present, this means it will require only a small amount of energy to remove particles from the liquid state to the gaseous state. These small molecules will be volatile substances and will evaporate easily. This is outlined in the image below:
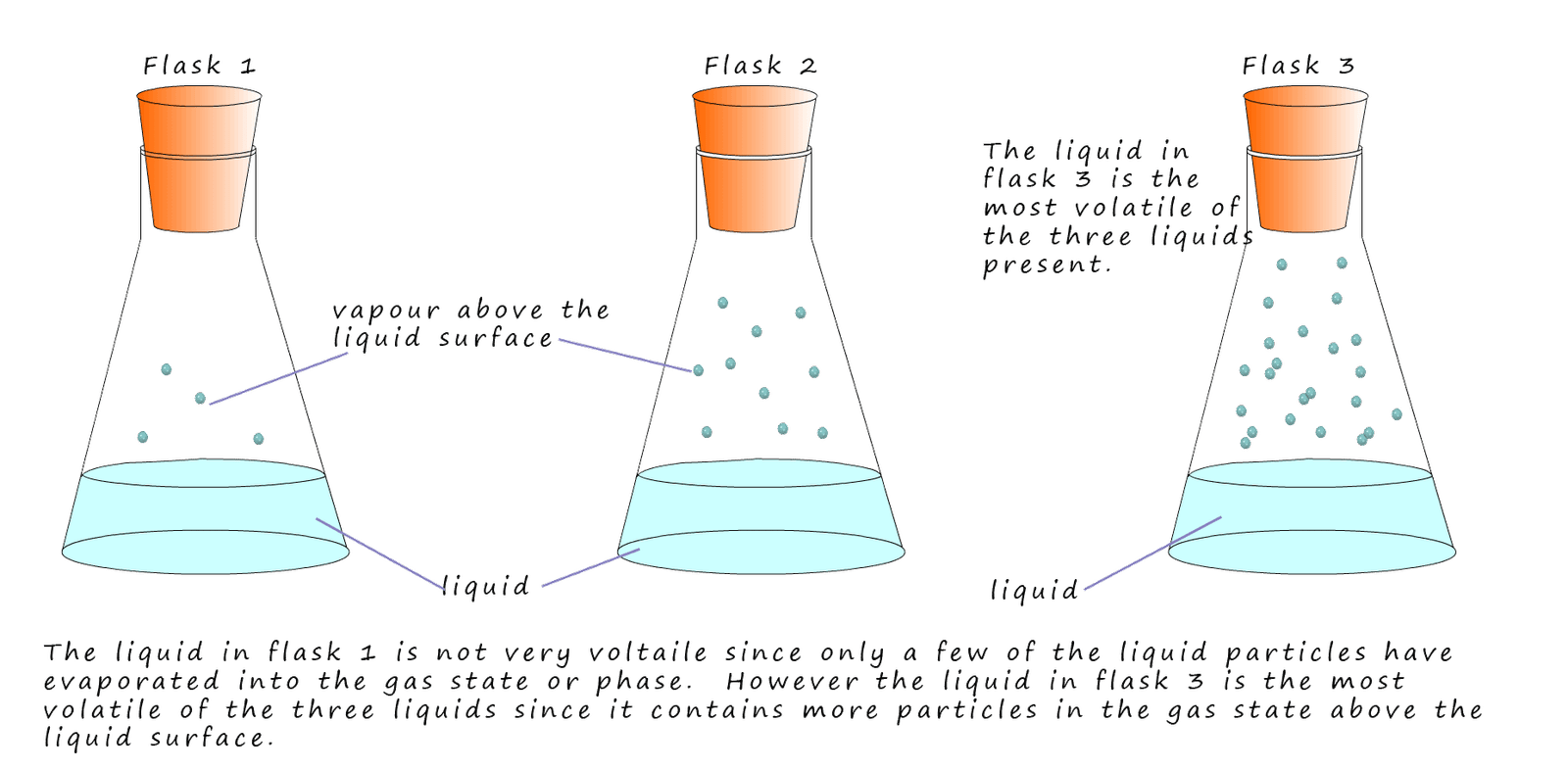
Alkanes which are liquids at room temperature and which contain small molecules will be runny; that is not particularly viscous, they will be volatile and evaporate easily. As the size of the alkane molecules increase then there will be more intermolecular bonding between the molecules and this along with their increase in mass will result in an increase in their viscosity and volatility.
 Alkanes are used mainly as fuels. Obviously if an alkane
is going to be used as a fuel it must
be flammable. But just how flammable are alkanes? Consider the following example:
Alkanes are used mainly as fuels. Obviously if an alkane
is going to be used as a fuel it must
be flammable. But just how flammable are alkanes? Consider the following example:
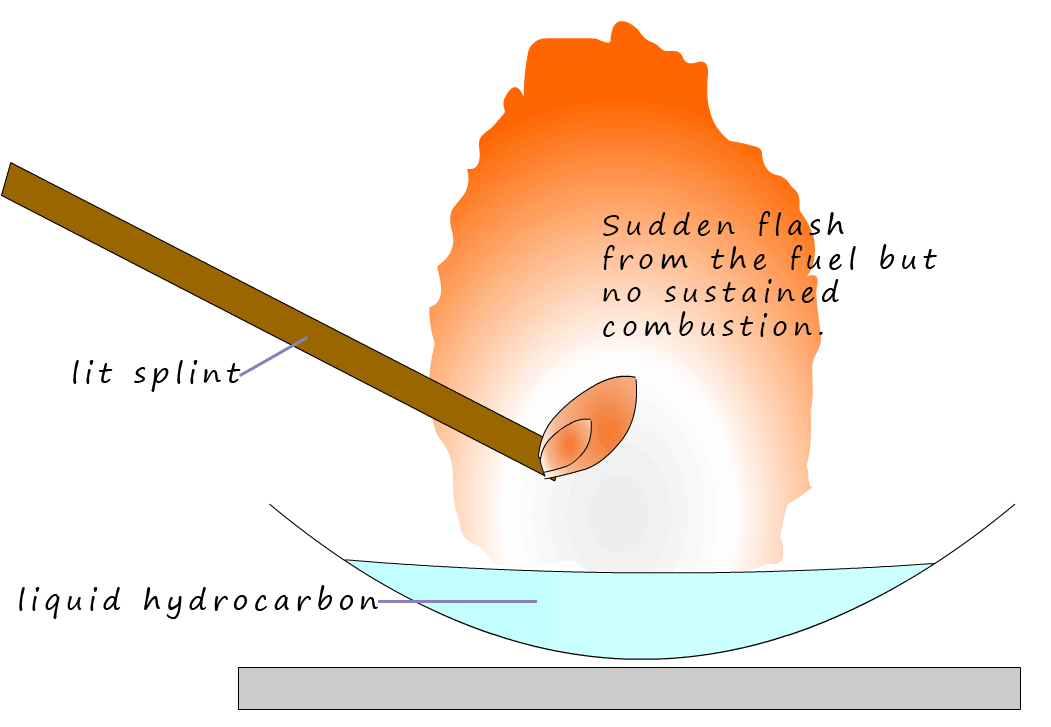
A liquid
is placed in an evaporating basin as shown
in the image opposite. If the liquid has a low boiling point it will be volatile, that is it will evaporate easily.
If it evaporates easily then above the liquid will be lots of vapour. If the liquid has a high boiling point it will not be
volatile and there will be very few vapour (gas) particles above the liquid. It is
the amount of
particles in the gas phase above the liquid surface that will determine how flammable it is. When a
lit splint is placed above a flammable liquid; it is not the liquid but the vapour above the liquid
which burns.
You may hear on TV drama shows such as CSI or fire fighters talking about the flash point of a substance. The flash point is
the point at which there is just enough vapour present above a liquid that when a flame is applied it will flash up
BUT there is not enough vapour to keep the flame going. The substance will not burn.
However the
fire point is the point at which there is enough vapour present to sustain combustion and the fuel
will continue to burn. Roughly speaking the fire point is about 100C higher than the flash point.
The lower the flash point the more flammable the fuel will be. The table below gives the flash points
for several alkane molecules.
| alkane | methane | propane | pentane | heptane | decane |
|---|---|---|---|---|---|
| flash point/oC | -188 | -104 | -49 | -7 | 46 |
From this table you can clearly see that small alkane molecules such as methane have very low flash points and that as the alkane molecules increase in size their flash and fire points will both increase due to the reduced volatility of the larger alkane molecules.
Match the terms below with their correct defintion by simply clicking on the term and then its correct defintion. Correct responses will turn the defintions green!
 The trend in the flash points and fire points are very clear, the larger the molecule the higher is its flash
point and hence the less flammable it will be. It is also worth noting that the smaller the
hydrocarbon molecule the more cleanly it burns. Large chain hydrocarbons are much more difficult to
ignite than smaller chain hydrocarbons and they burn with very dirty sooty flames. You may also hear
people refer to incomplete combustion as dirty combustion because of the soot it produces. You can
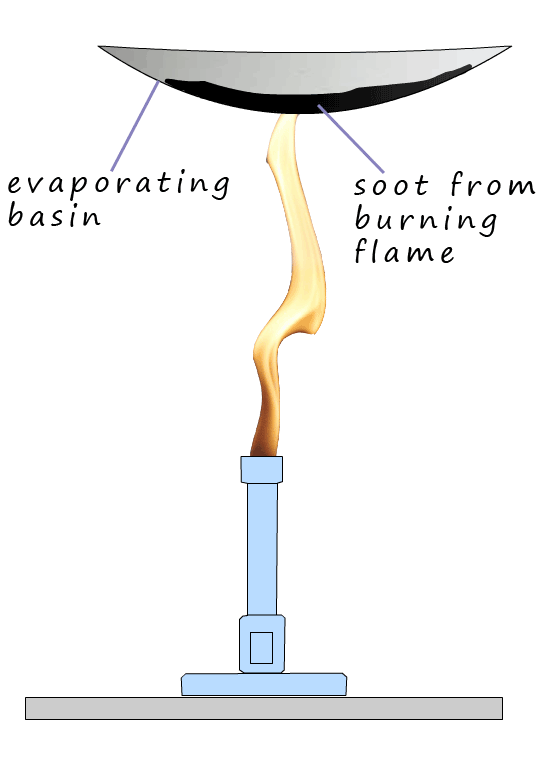
easily see the soot in a flame by placing a ceramic cup or in the image shown opposite a clean white evaporating
basin in a Bunsen flame and after a few seconds the nice clean evaporating basin is black with soot from the dirty Bunsen flame.
The trend in the flash points and fire points are very clear, the larger the molecule the higher is its flash
point and hence the less flammable it will be. It is also worth noting that the smaller the
hydrocarbon molecule the more cleanly it burns. Large chain hydrocarbons are much more difficult to
ignite than smaller chain hydrocarbons and they burn with very dirty sooty flames. You may also hear
people refer to incomplete combustion as dirty combustion because of the soot it produces. You can
easily see the soot in a flame by placing a ceramic cup or in the image shown opposite a clean white evaporating
basin in a Bunsen flame and after a few seconds the nice clean evaporating basin is black with soot from the dirty Bunsen flame.
Answer the two questions below to review your understanding of a few of the key terms covered so far, click the blue box to reveal the answer.:
 As the molecular size of alkane molecules increase, their boiling points generally increase. This is because larger alkane molecules have a greater molecular mass and crucially, more intermolecular bonding between individual molecules. More energy is therefore required to overcome these stronger intermolecular forces, leading to higher boiling points.
As the molecular size of alkane molecules increase, their boiling points generally increase. This is because larger alkane molecules have a greater molecular mass and crucially, more intermolecular bonding between individual molecules. More energy is therefore required to overcome these stronger intermolecular forces, leading to higher boiling points.
 Volatility refers to how easily a substance evaporates. Smaller alkane molecules are more volatile than larger ones. This is because they have lower boiling points and less intermolecular bonding, meaning less energy is required to convert them from a liquid to a gaseous state. An example small alkane molecules like methane (boiling point -161°C) are highly volatile, while larger alkane molecules such as decane (boiling point 174°C) are less volatile.
Volatility refers to how easily a substance evaporates. Smaller alkane molecules are more volatile than larger ones. This is because they have lower boiling points and less intermolecular bonding, meaning less energy is required to convert them from a liquid to a gaseous state. An example small alkane molecules like methane (boiling point -161°C) are highly volatile, while larger alkane molecules such as decane (boiling point 174°C) are less volatile.